|
|
Institute of Biochemistry and Molecular Genetics Vrazov trg 2 Tel (IBK): +386-1-543-7640 |
|
|
|
|
Laboratory for Extracellular Vesicle Research
|
||
|
HOME I
MEMBERS I
RESEARCH I
RESEARCH SUPPORT I
TALKS & RECOGNITIONS I
EQUIPMENT I
LINKS I
|
||
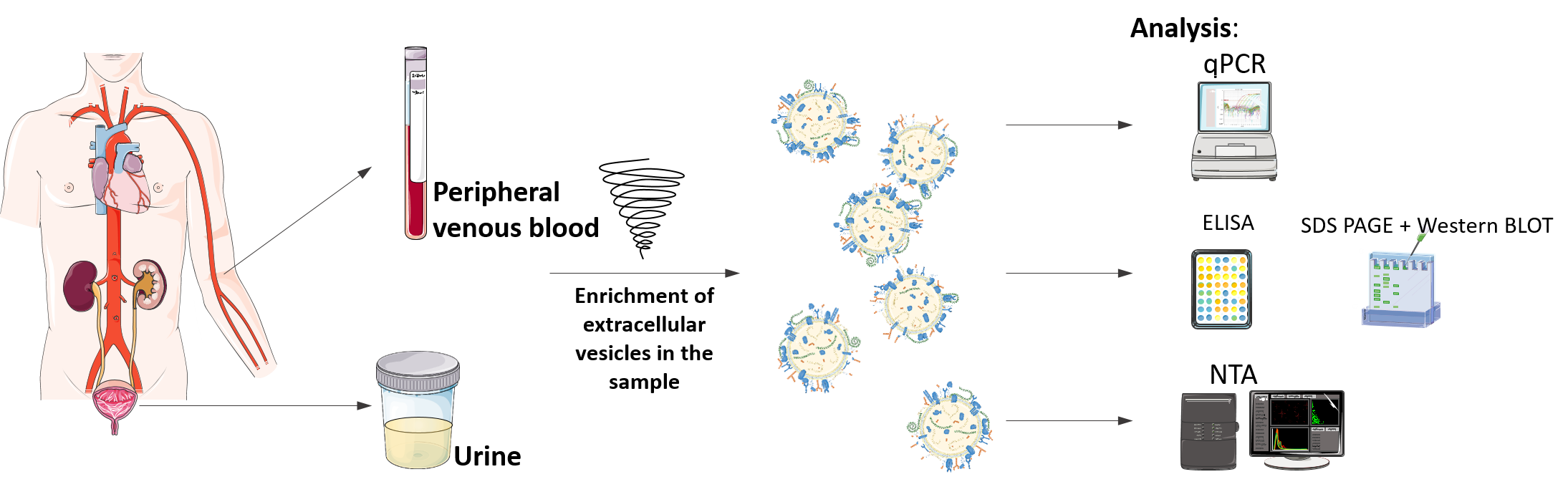
Extracellular vesicles as biomarkers of disease
(1) We have established workflows for (i) collection and storage of blood plasma and urine that is compatible with EV research, (ii) isolation of EVs from blood plasma and urine and (iii) characterization of EV size/concentration, RNA, DNA and protein content. We are applying them in several ongoing collaborative biomarker studies with clinical partners on diverse diseases like HIV-1 infection, kidney allograft rejection, diverse cancers. (2) We are also directly involved in ISEV society efforts in promoting Rigor and Standardization in EV research by co-authoring guidelines, reports and recommendations. |
||